
Reproductive Health
Noninvasive prenatal genetic testing technology is a new type of fetal chromosome disease technology. It has the advantages of safe, accurate, simple and rapid. Through collecting the venous blood of pregnant women, we can extract the plasma free DNA. Using high-throughput sequencing technology to judge whether pregnant women with chromosomal aneuploid fetal. The technique can be tested after 12 weeks of pregnancy. The accuracy is much higher than traditional serological screening, and it can avoid the fetal intrauterine infection and miscarriage risk of prenatal diagnosis. It is of great significance to reduce the birth defect rate and improve eugenics.
Product introduction:
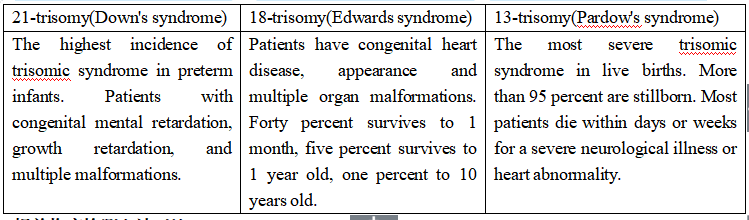
The free DNA of the fetus can be detected in the peripheral blood of pregnant women after 4 weeks. The concentration of free DNA of the fetus increases with the number of pregnant weeks. After 12 weeks of pregnancy, by extracting cell-free fetal DNA in peripheral blood (10 ml) of pregnant women, using high-throughput sequencing technology and bioinformatics analysis method, we can accurately determine whether the fetus with chromosomal aneuploid disease, including: 21-trisomy, 18-trisomy and 13-trisomy. This method has the characteristics of early, noninvasive, high sensitivity and high accuracy.
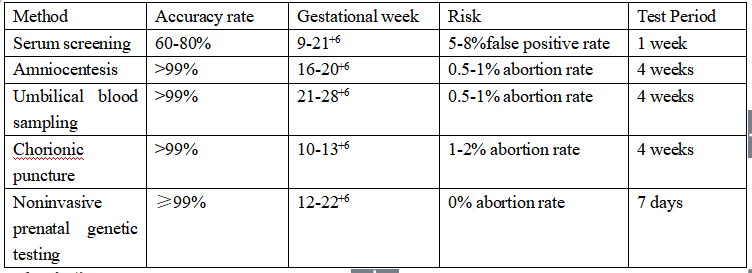
Comparison of related clinical detection methods
Intended for
1. Serological screening and imaging examination revealed the critical risk of common chromosome aneuploidy.
2. Contraindications of interventional prenatal diagnosis, such as threatened abortion, fever, hemorrhagic tendency, activity period of chronic pathogen infection, pregnant women with RH negative blood type and so on.
3. More than 20+6 weeks of pregnancy, pregnant women missed the best time of serological screening, or missed the routine prenatal diagnosis, but have the demand of evaluating the risk of 21-tribody syndrome, 18-trisomal syndrome, 13-trisomal syndrome.
Detection process
Flow 1.Fill in the test application form and sign a consent form.
Flow 2. Extract 10Ml venous blood of pregnant women.
Flow 3. Perform Noninvasive prenatal genetic testing.
Flow 4. Issue the low test risk report. Continue routine pregnancy testing.
Issue the high test risk report. Genetic counseling and prenatal diagnosis.
Sampling notes and reporting cycles
1. Non-invasive prenatal testing is as the same as normal venous blood sampling, and we need 10 ml blood of pregnant women.
2. The extraction of blood does not need an empty stomach. No prior inspection is required, pregnant women only need to have the normal diet and rest.
3. Issuing the non-invasive prenatal testing report within 7 days.
Technical advantage
Safe: Only need 10 ml blood of pregnant women. Non-invasive. No miscarriage. No infection.
Accurate: The new generation of gene sequencing detection rate is over 99.9%.
Early: Testing after 12 weeks. Finding early problems and taking measures.
Fast: Issuing the non-invasive prenatal testing report within 7 days.
Be careful:
Noninvasive prenatal gene detection could not replace amniocentesis of fetal chromosomes.
- Noninvasive prenatal gene detection
- Genetic testing for hereditary deafness
- Neonatal Genetic Metabolic Disease
- Chromosomal Abnormality
- Metabolic Capacity Of Folic Acid
- Detection of microdeletion of chromosome microdeletion
- Chromosome microarray (CMA)
- Detection of children's safety in drug use
Address: No. 1109, Hsing three road, Ji'nan high tech Zone, Shandong
Phone: 400-966-22200
Web site: www.yinfenggene.com
Mailbox::yinfengyixuejianyan@yfyxjy.com

